What Best Describes How an Ionic Bond Forms
Describe the general properties of. The correct option is A.

Ionic Compounds Bonds Structure Properties 1 6 5 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams
One atom pulls an electron from another atom.
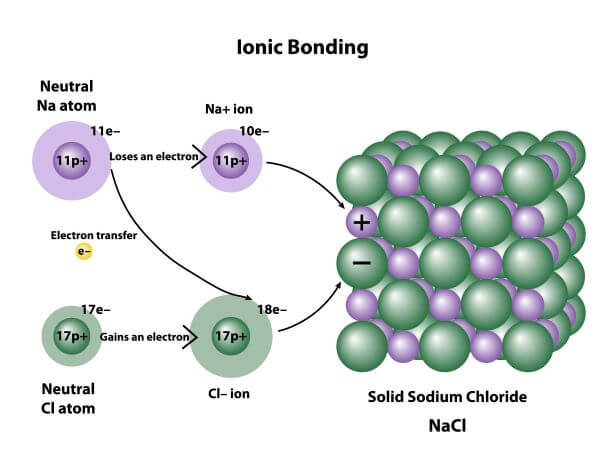
. Two molecules share an electron. This type of bonding leads to the formation of two oppositely charged ions positive ion known as cations and negative ions are known as anions. A metal and nonmetal react to form an ionic bond.
--The formula of ammonium fluoride is NH4F. A In ionic bonds electrons are shared while in covalent bonds electrons are ripped between atoms. Which statement best describes how an ionic bond forms.
An electromagnetic attraction that occurs between two metals. Two atoms both lose an electron. Ions of opposite charges are formed in the reaction of a metal and a.
The question stateswhich statement best describes how an ionic bond forms. Which statement about crystal lattice energy is best supported by the information in the table. A force that holds two oppositely charged ions together.
The transfer of electrons forms strong bonds between ions. The correct option is A. A charged particle that forms when an atom or group of atoms gains or loses one or more electrons.
Which best describes how an ionic bond forms. The atom that donate the electron become a positively charged ion while the atom that received the atom become a negatively charged ion. The transfer of electrons forms strong bonds between ions.
The sharing of electrons forms strong bonds between. This bond is the result of the nonmetal desiring electrons to fill out its valence and a metal. Question 1 1 pts Which statement best describes ionic and covalent bonds.
Two atoms share valence electrons B. --The atoms in each ion are bonded together covalently to form a single unit. Which of the following best describes how a liver cell and skin cell have the exact same DNA sequence and yet look different and perform different fun.
An Ionic bond is the bond formed by the complete transfer of valence electron to attain stability. The question sayswhich statement best describes how an ionic bond forms. --The formula of potassium sulfate is K2SO4.
Two molecules share a Get the answers you need now. Ionic bonds are formed when positively charged ions and negatively charged ions come together in a lattice. Which property is best to use when determining the strength of an ionic bond in a solid.
What best describes how an ionic bond forms. Ionic bonds are formed as a result of complete transfer of electrovalence electrons from one atom to another. The presence of two oppositely charged ions results in a strong attractive force between them.
Which pair of elements will form an ionic bond. The regular pattern in which a crystal is arranged. The table lists the lattice energies of some compounds.
A force that holds two oppositely charged ions together. The attractive force between oppositely charged ions which form when electrons are transferred from one atom to another. Explain what lattice energy is and how it affects the properties of ionic compounds.
Biology 19092019 0620 lisnel Which best describes how an ionic bond forms. --The charge is distributed over the entire ion. Ionic bonds are formed as a result of complete transfer of.
Which statement best describes how an ionic bond forms. Which statement best describes how an ionic bond forms. Which best describes how an ionic bond forms.
The transfer of electrons forms strong bonds between ions. B In ionic bonds and covalent bonds electrons are shared.

What Are Ionic Compounds And How They Are Formed

Ionic Bond Definition Properties Examples Facts Britannica

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
Comments
Post a Comment